
What is a Microthane Prosthesis?
The silicone Microthane prosthesis' surface, covered in polyurethane, is specially treated and covered by a fine mesh and designed to grow surrounding tissue inside, to reduce side effects such as the prosthesis moving around and rotating as well as reducing the possibility of capsular contracture. Also, the bond between the prosthesis and tissue quickly progresses, resulting in a fast recovery and natural shape and feel.
Microthane + MINE HD Endoscopic
Microthane
+
HD Endoscopic Breast Augmentation
The collaboration of the premium Microthane prosthesis and Endoscopic HD Breast Augmentation, filled with MINE's breast augmentation know-how, to complete a beautiful breast line that is best suited to me.
Microthane
Special Features of the Microthane prosthesis
01
Reducing probability of capsular contracture,
movement and rotation of the prosthesis
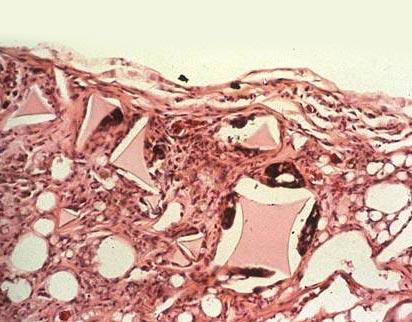
Because the surface of the prosthesis is covered in a fine mesh, the vascular and tissue cells grow inwards inside of the mesh with no pressure on the prosthesis, resulting in a notieceably lower probability of capsular contracture.
What is Capsular Contracture ?
When foreign substances enter the body, the body raises its level of immunity and form fiber cells producing collagen fibers. These collagen fibers surrond the prosthesis and create a film called a capsule. This is a natural occurence, but in the case where the periprosthetic capsule (capsule of scar tissue) becomes overly thick, due to various causes such as a bad reaction to the prosthesis, infection, or bleeding, the prosthesis hardens and forms, what we call, capsular contracture.
02
The feel and movement similar to
natural breasts

Because the surface of the prosthesis is covered in a fine mesh, the surrounding tissue grows inside and safely combines with the body's tissue. The strength of the periprosthetic capsule (capsule of scar tissue) is dispersed, inhibiting the formation of the periprosthetic capsule, making the feel and shape smooth and natural.
03
Quick Recovery and Return to Daily Activities
In comparison to preexisting prostheses, new blood vessels rapidly form and new reactions are improved. Therefore, the inserted prosthesis safely stabilizes in a short period of time leading to a quick recovery.
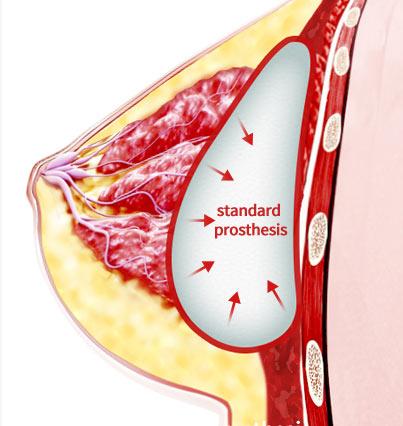
Standard Prosthesis
- development of new blood vessels is slow due to development of encapsulation
- The inserted prosthesis needs time to stabilize
- Difficult to return to daily activities immediately after surgery
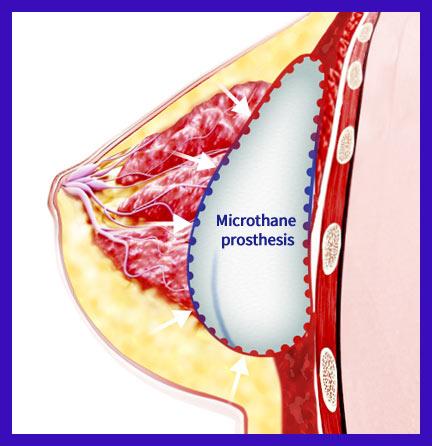
Microthane Prosthesis
- New blood vessels rapidly form due to improved vascularization response
- The inserted prosthesis stabilizes in a short period of time
- Possible of quick recovery to daily activities even over a short break
04
Strong against external impact Safe due to the existence of a barrier layer
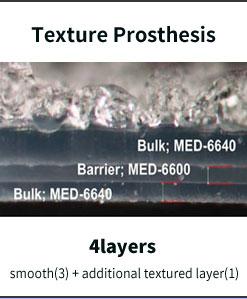
In preexisting prostheses it is normal to create a hole in the surface for texturing, but the Microthane prosthesis does not create a hole, but creates another layer by covering the
surface with polyurethane, making it strong against external impact with almost no chance of rupturing. It is safe as there are 5 layers consisting of the barrier layer, which blocks the leakage of charged gel inside the prosthesis.
The difference from preexisting prostheses

VS

VS



Safety confirmed through clinical cases of renowned overseas medical personnel
Microthane Prostheses with numerous clinical cases
1988
Hester
Clinical and research literature and current interests – Discussion of action mechanism and action impacting the body, potential risks of TDA, as well as toxicity and infection rate >total capsular contraction occurence rate:2%(expanded)
Clinical time period 1983~1988 (4.3 years)
Clinical target 1199 polyurethane implants
1992
Gasperoni
Produces very good results with regard to breasts contour and density through 12 years of experience using Polyurethane-covered artificial breasts>total capsular contraction occurence rate: 3.3%
Clinical time period 1979~1991 (12 years)
Clinical target total 400 implants 306 Memes 91 Replicons Y3 naturals
1997
Hester
A measurement of 2,4TDA from women's urine and serum samples of Meme or Replicon artificial breast product treatments>confirmed safety wiht respect to TDA
Clinical target 66 meme replicon polyure-thane implants (polytech)
1997
Cohney
Presentation of an improved removal method of Polyurethane Foam-Covered Gel artificial breast>After 5-10 years of use, the probability of capsular contracture occurence was much smaller than that of a similar artificial breast "Slick", natural movement
1999
Va´zquez
With the use of polyurethane-covered silicone gel breast implants,lower rates of incidence of capsular contracture was confirmed
Clinical time period 10 years
Clinical target total 811 implants 24 memes 6 Replicons 781 silimeds
2000
Vogetti
Presentation of an improved removal method of Polyurethane Foam-Covered Gel artificial breast>After 5-10 years of use, the probability of capsular contracture occurence was much smaller than that of a similar artificial breast "Slick", natural movement
Clinical time period -
Clinical target total 14 implants
2005
Salgarello
Reporting experience carried out over the last 24 months using the best anatomical artificial breast in immediate breast restoration following SSM>Aesthetic results are considered good or excellent by surgeons and patients in more than 90% of cases.
Clinical time period -
Clinical target total 14 implants
2006
Handel
Evaluate the long term experiences of the polyurethane foam-covered implants and compare with the results and probability of complications of other types of artificial breast prosthetics.
Clinical time period 1981~2004(23years)
Clinical target total 1531 Implants 345 memes 618 Textured implants 568 Poly urethane implants
2006
Salgarello
Reporting experience carried out over the last 24 months using the best anatomical artificial breast in immediate breast restoration following SSM>Aesthetic results are considered good or excellent by surgeons and patients in more than 90% of cases.
Clinical time period 1979~2004(25years)
Clinical target total 1055 implants 488 smooth implants 566 polyurethane implants





